What Molecule Does Hemoglobin Decay Into Over Time
Oxygen does not bind to Fe3. What is usually but not always related to the.

Genetic Variation Influencing Hemoglobin Levels And Risk For Anemia Across Populations Barrera Reyes 2019 Annals Of The New York Academy Of Sciences Wiley Online Library
Do plant cells perform cellular respiration.

. Do you know the correct answer. In order to achieve 25 saturation an average of 1 O 2 molecule per hemoglobin the. This process also produces one molecule of carbon monoxide for every molecule of heme degraded.
Biology 22062019 1420. So the population decreased by 24 because 33810226-224. In the absence of 23-BPG hemoglobin behaves like myoglobin which becomes half-saturated at only 1 Torr.
What molecule does hemoglobin decay into over time. Why does controlling transcription affect prokaryotic gene expression. You know the right answer.
Globular proteins are broken down into amino acids heme is converted to biliverdin and iron is removed What is the fate of hemoglobin that has not been phagocytized. What molecule does hemoglobin decay into over time. An enzyme known as diaphorase is responsible for converting methemoglobin back into hemoglobin.
What molecule does hemoglobin decay into over time. It is stored in liver and reused to form enzymes myoglobin hemoglobin etc. What molecule does hemoglobin decay into over time.
Which phase of mitosis is the cell most likely in. When an adp molecule gains a phosphate it becomes an atp molecule. Correct answer to the question 12.
However the conversion is a natural process that happens over time. Explain why the Blue People of Troublesome Creek were blue give as much detail as possible. A cell is viewed under a microscope and is found to have two nuclear envelopes and spindles that appear to be breaking apart.
Primary consumers should come after the sun4. Explain why the Blue People of Troublesome creek were blue in as much detail as possible. Further evidence for the flexibility of proteins can be obtained by noting that there is no path in the crystal structures of myoglobin and hemoglobin along which an O 2 molecule can travel to reach the.
Which of the organisms would most likely survive the change to their environment. The answer is that inside each hemoglobin molecule oxygen molecules attach to waiting atoms of iron. You have probably seen what happens when oxygen and iron get together in the presence of water.
Up to 24 cash back What molecule does hemoglobin decay into over time. When iron rusts the oxygen is locked up permanently in a crystal. When not bonded to O2 deoxyhemoglobin stays in a tensed state or conformation.
How will genetic drift affect this population. Which of the following are signs and symptoms of dengue fever. Secondary consumers should be at the bottom of the pyramid2.
The result is usually iron oxide rust. Up to 24 cash back What molecule does hemoglobin decay into over time. What molecule does hemoglobin decay into over time.
A the sequence of bases would halt the production of any proteins. Under physiological conditions hemoglobin is half-saturated with oxygen at pO2 of 26 Torr. So how is it that all our hemoglobin doesnt just turn into methemoglobin.
Provided by hemoglobins cooperativity in oxygen binding. Because it has four subunits a hemoglobin molecule can reversibly bond with up to four O2 molecules. Why is this cure ironic or why is this cure funny.
What molecule does hemoglobin decay into over time. Without it hemoglobin would be an inefficient transporter releasing only about 8 of its cargo in the tissues. This is reused for whatever protein is required for.
What molecule does hemoglobin decay into over time. What was the cure for methemoglobinemia. Biology 22062019 1330 lizredrose5.
What molecule does hemoglobin decay into over time. How is this molecule different from hemoglobin. Up to 24 cash back If all of our hemoglobin were converted to methemoglobin it would be useless.
What molecule does hemoglobin decay into over time. The hemoglobin breaks down and the alpha and beta chains are eliminated in urine. What was the cure for methemoglobinemia.
The first O2 molecule to bond causes the oxyhemoglobin to shift to a relaxed state. What is the role of the enzyme diaphorase. Energy retained should.
The sun has an arrow leading to decomposers 3. It is a protein that is further broken into amino acids. What molecule does hemoglobin decay into over time.
In other words the binding of one O 2 molecule affects the binding of others as we can see by the following. How is this molecule different from hemoglobin. Protein phosphorylation is commonly involved with all of the following except.
Asmall population of chimpanzees lives in a habitat thant undergoes no change for a long period of time. This process is called Other questions on the subject. What is the role of the enzyme diaphorase.
Hemoglobin Kirklareli A H58l A New Variant Associated With Iron Deficiency And Increased Co Binding Journal Of Biological Chemistry 1 Show answers Another question on Biology. Within these cells the hemoglobin molecule is broken up and the iron gets recycled. Overtime the pond slowly becomes more acidic due to the release of chemicals from a nearby factory.
Biology 22062019 0630 poohnia. What molecule does hemoglobin decay into over time. Population growth is decreasing-2 4-10-3-8-3.
Hemoglobin efficiency without 23-BPG. The changes that occur in the structure of hemoglobin when oxygen binds to the hemes are so large that crystals of deoxygenated hemoglobin shatter when exposed to oxygen. How is this molecule different from hemoglobinThis is because methemoglobin has a slightly different ion of iron different than that of hemoglobin it has one less electron and it.
Whats wrong with this ecological pyramid. Haemoglobin is broken down into its three parts. So how does the hemoglobin molecule.
The remaining part forms bilirubin which is excreted via liver.
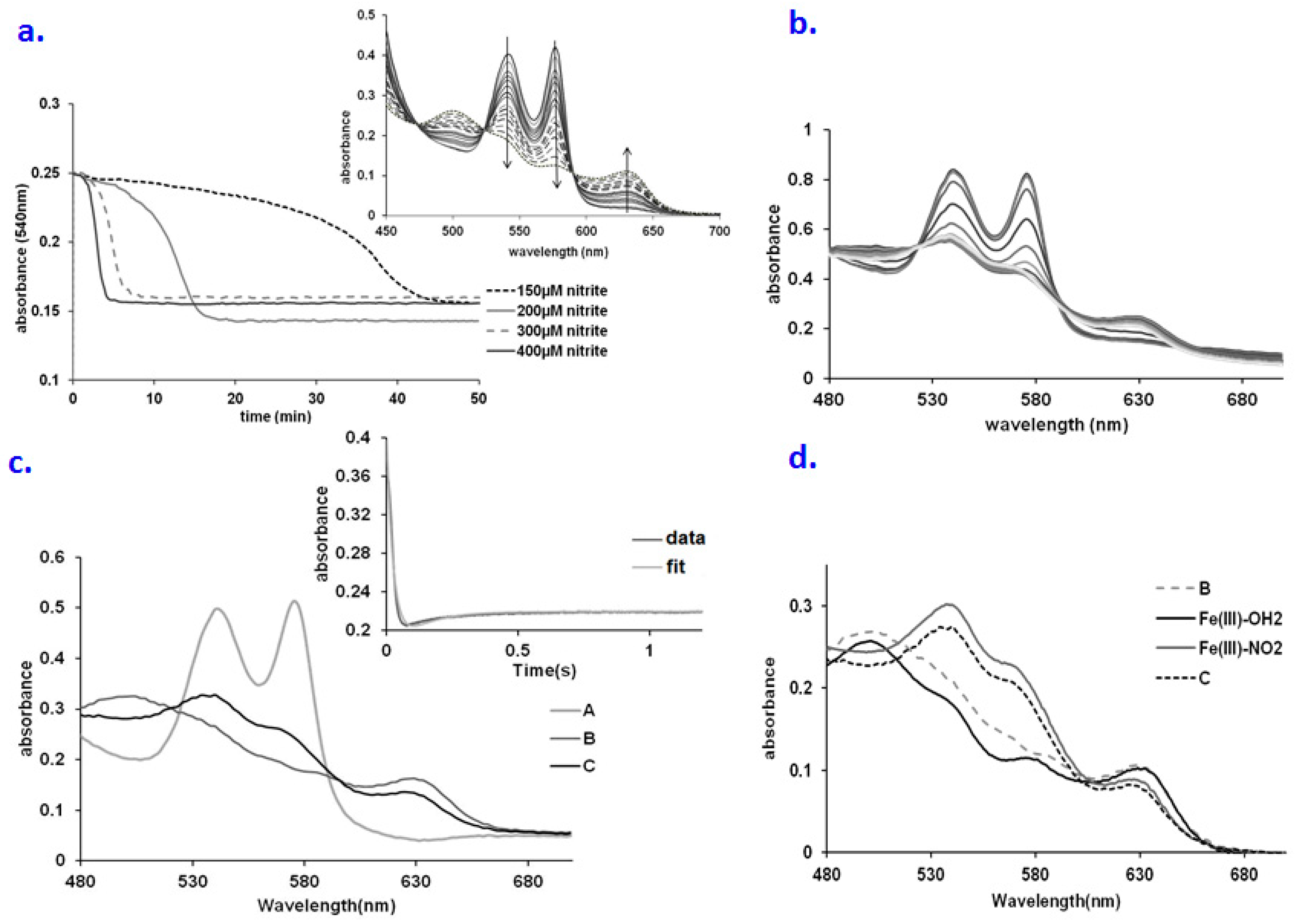
Molecules Free Full Text The Reaction Of Oxy Hemoglobin With Nitrite Mechanism Antioxidant Modulated Effect And Implications For Blood Substitute Evaluation Html

Oxyhemoglobin An Overview Sciencedirect Topics

Hemoglobin Kirklareli A H58l A New Variant Associated With Iron Deficiency And Increased Co Binding Journal Of Biological Chemistry

Probing The Hemoglobin Central Cavity By Direct Quantification Of Effector Binding Using Fluorescence Lifetime Methods Journal Of Biological Chemistry
Comments
Post a Comment